Beauty is an eternal pursuit of all people.
Exosomes are the main demands for medical aesthetics
At present, the repair and remodeling of human body shape and function in medical aesthetics are often achieved by injection of antioxidants, hyaluronic acid and botulinum toxin, stem cell therapy. However, there are some challenges, such as difficulty controlling the injection dose, abnormal local contour, increased foreign body sensation, and the risk of tumor occurrence and deformity induced by stem cell therapy. Exosomes are tiny vesicles secreted by cells, which are rich in proteins, nucleic acids and other bioactive molecules. They have the characteristics of low immunogenicity and strong tissue penetration, making them ideal for applications in medical aesthetics. However, their low yield, strong heterogeneity, and long-term preservation still hinder their application in medical aesthetics. In this review, we summarize the mechanism of action, administration methods, engineered production and preservation technologies for exosomes in medical aesthetics in recent years to further promote their research and industrialization in the field of medical aesthetics.
1. Introduction
Beauty is the eternal pursuit of all people. Wound repair (Prasai et al., 2022), anti-aging (Hu et al., 2019), inhibiting hyperpigmentation (Wang et al., 2021) and inhibiting hair loss (Yang G. et al., 2019) are the main demands for medical aesthetics. Medical aesthetics is a perfect combination of regenerative medicine and improvement of one’s looks (Edmonds, 2013). It repairs, replaces, or regenerates human cells and tissues by using regenerative medicine technologies such as cells, natural or artificial scaffold materials and growth factors (Lee J. et al., 2020; Huang et al., 2021). At the same time, it is used in medical aesthetics to achieve the repair, remodeling and improvement of human appearance, shape and function, so as to achieve the harmony and improvement of aesthetics, medicine, shape and function of the human body (Martínez-González et al., 2019; Xiong et al., 2021).
At present, in the field of medical aesthetics, anti-oxidants such as vitamin C (Odrobinska et al., 2020) and resveratrol (Ratz-Lyko and Arct, 2019), hyaluronic acid (Shekhter et al., 2019; Gazitaeva et al., 2021) and botulinum toxin (Naik, 2021) are often injected to remove wrinkles, reduce color spots and promote wound healing. However, there are still many challenges in aesthetics, for example, it is difficult to control the dose of anti-oxidant injection (Shekhter et al., 2019; Gazitaeva et al., 2021). In clinical practice, fat transplantation is often used in medical aesthetics, but it may lead abnormal local contour shape and to increase foreign body sensation (Yan et al., 2021). Subsequently, stem cell therapy was gradually applied to regenerative medicine due to its pluripotency, self-renewal and ability to promote the secretion of regenerative cytokines (Johari et al., 2019; Li W. et al., 2022), but stem cell therapy may induce the risk of tumorigenesis and malformation (Trounson and McDonald, 2015). Moreover, exosomes are nano vesicles secreted by cells, which belong to one kind of extracellular vesicles and play an important role in intercellular communication (Mehryab et al., 2020). Compared with drugs, plant/herbal extracts and bioactive molecules, exosomes have the advantages of low immunogenicity, good biocompatibility, targeting specificity and strong tissue permeability (Ha et al., 2016; Liang et al., 2021; Rao et al., 2021). They are often used as drug delivery vehicles and can play a role in medical aesthetics (Li M. et al., 2020). Here, we summarize the mechanism of action, administration methods, engineered production, separation, purification and presser-vation methods of exosomes in the field of medical aesthetics in recent years with a view to furthering the research and industrialization process of exosomes in the field of medical aesthetics.
2 Biological properties of exosomes
Exosomes have unique physicochemical properties, such as their ability to pass through tissue barriers, mononuclear phagocytic cell systems and certain targeting properties (Marcus and Leonard, 2013) when carrying drugs, and they are often used as therapeutic drug delivery carriers in medical aesthetics. For example, exosomes’ lipid bilayer structure can prolong drug circulation time in vivo, escaping the elimination by mononuclear phagocytosis system, increasing the local drug concentration, and effectively controlling the drug release (Nam et al., 2020). Compared with traditional nanomaterials, exosomes have good biocompatibility, degradability, low toxicity, and low immunogenicity, so they are more suitable as drug delivery carriers (O'Brien et al., 2020).
2.1 Structure and composition of exosomes
The lipid bilayer membrane structure of exosomes protects the abundant proteins, nucleic acids, microRNAs (miRNAs), cholesterol and sphingomyelin in the membrane from being degraded (Pegtel and Gould, 2019; Li X. et al., 2020; Foo et al., 2021; Gurunathan et al., 2021) (Figure 1). A common cytoplasmic protein in exosomes is the RAB protein, a member of the guanylate triphosphatase (GTPases) family, which regulates the fusion of exosome membranes with recipient cells (An et al., 2021). In addition to RAB proteins, exosomes are rich in annexins that have exosomal membrane exchange and fusion effects (Tan et al., 2017; Keklikoglou et al., 2019). Exosomes membrane is rich in tetraspanins involved in exosomes transport family (CD63, CD81, and CD9) (Barranco et al., 2019) and heat shock protein family (HSP60, HSP70, and HSP90) (Regimbeau et al., 2021). Exosomes transport cargoes through the lipid bilayer membrane, and can deliver active ingredients (including proteins, nucleic acids, and lipids) from parent cells to recipient cells (Villarroya-Beltri et al., 2014; van Niel et al., 2018), and they can selectively enter target cells (Li M. et al., 2020) by homing to target tissues. Their active ingredients are delivered to the target cells’ cytoplasm, thereby changing recipient cells’ physiological state (Tkach and Thery, 2016).
2.2 Biogenesis
In the aspect of medical aesthetics, the research of exosomes biogenesis is crucial to the engineering transformation of exosomes and the production of larger quantities. A short overview of exosomes biogenesis can be stated as the inward movement of cytoplasmic membranes results in wrapping around extracellular entities and various membrane proteins forming the early sorting endosomes (ESEs), the ESEs fuses with other ESEs to form late sorting exosomes (LSEs). The LSEs develop into multivesicular bodies (MVBs). MVBs contain many intraluminal vesicles (ILVs) that are released into exosomes. After its formation, the MVBs can either be degraded by fusion with lysosomes or fuse with the plasma membrane with ILVs in it, the final exosomes (Borges et al., 2013; Matic et al., 2020; Ras-Carmona et al., 2021).
Because the biogenesis of exosomes is mainly divided into two ways: dependent on the transport of necessary endosome sorting complex (ESCRT) pathway and independent of ESCRT. Therefore, we herein discuss some ESCRT-dependent and ESCRT-independent mechanisms to increase exosome production (Figure 2). These following methods provide important ideas on engineering preparation of related exosomes in the field of later medical aesthetics and improve their production rate. ESCRT-dependent pathways: Genetic manipulation of gene generation pathways to overexpress activating genes for exosome biogenesis and downregulating key regulatory genes involved in exosome transport, storage, secretion, or recycling. In most of these pathways, genes have a directly positive effect on exosome production (Jafari et al., 2020). For example, Wang et al. found that overexpression of HSP20 attenuated diabetes-induced cardiac damage. In addition, the elevation of HSP20 promoted the secretion of exosomes by directly interacting with Tsg101, a promoter of the exosome biogenesis pathway, and the production of exosomes was increased by 1.8-fold compared with the control group (Wang et al., 2016) (Table 1 for details).
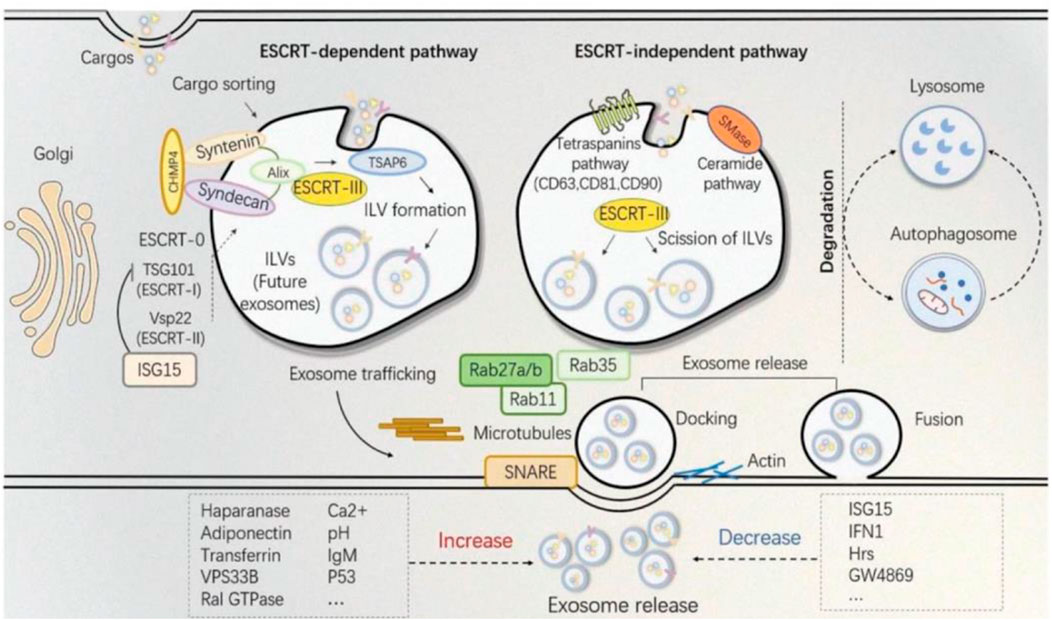
FIGURE 2. The established theory of exosomes biogenesis indicates different pathways distinguished as ESCRT-dependent and ESCRT-independent (Shi et al., 2021).
2.2 Biogenesis
In the aspect of medical aesthetics, the research of exosomes biogenesis is crucial to the engineering transformation of exosomes and the production of larger quantities. A short overview of exosomes biogenesis can be stated as the inward movement of cytoplasmic membranes results in wrapping around extracellular entities and various membrane proteins forming the early sorting endosomes (ESEs), the ESEs fuses with other ESEs to form late sorting exosomes (LSEs). The LSEs develop into multivesicular bodies (MVBs). MVBs contain many intraluminal vesicles (ILVs) that are released into exosomes. After its formation, the MVBs can either be degraded by fusion with lysosomes or fuse with the plasma membrane with ILVs in it, the final exosomes (Borges et al., 2013; Matic et al., 2020; Ras-Carmona et al., 2021).
Because the biogenesis of exosomes is mainly divided into two ways: dependent on the transport of necessary endosome sorting complex (ESCRT) pathway and independent of ESCRT. Therefore, we herein discuss some ESCRT-dependent and ESCRT-independent mechanisms to increase exosome production (Figure 2). These following methods provide important ideas on engineering preparation of related exosomes in the field of later medical aesthetics and improve their production rate. ESCRT-dependent pathways: Genetic manipulation of gene generation pathways to overexpress activating genes for exosome biogenesis and downregulating key regulatory genes involved in exosome transport, storage, secretion, or recycling. In most of these pathways, genes have a directly positive effect on exosome production (Jafari et al., 2020). For example, Wang et al. found that overexpression of HSP20 attenuated diabetes-induced cardiac damage. In addition, the elevation of HSP20 promoted the secretion of exosomes by directly interacting with Tsg101, a promoter of the exosome biogenesis pathway, and the production of exosomes was increased by 1.8-fold compared with the control group (Wang et al., 2016) (Table 1 for details).